Abstract
Background:Antiphospholipid Syndrome (APS) is associated with clinical thrombosis manifestations. However, it is characterized as prolongation of lag time in phospholipid (PL) dependent laboratory coagulation assays, a reflection of the paradoxical lupus anticoagulant (LAC) effect. In contrast to clinical laboratory testing which almost exclusively utilize synthetic PL (sPL), activated platelets are a major source for PL for in vivo hemostasis. It was recently reported that platelet-derived microvesicles (P-MVs) release is mediated by a positive thrombin generation feedback loop in the process of platelet activation and membrane ballooning, which supports a physiological role for P-MVs in hemostasis (Agbani, et. al., 2017).
Objective: To evaluate and compare thrombin generation (TG) supported by P-MVs, Erythroid MVs (E-MVs) and sPL in normal subjects and APS patients.
Methods: MVs were prepared by gentle sonication or hypotonic-mechanical disruption of washed whole blood platelet pellets, platelet apheresis units or red blood units; sPL composition used was PS/PE/PC=20/20/60 (Bloemen, et. al., 2017). Particle size distribution and anionic phosphatidylserine (PS) expression on P-MV, E-MVs and sPL by Annexin 5-Oregan green (A5-OG) staining were analyzed by flow cytometry using Apogee A60-Micro Plus. TG measured by calibrated automated thrombinography (CAT) was used to evaluate a procoagulable capacity of sPL, P-MVs, and E-MVs after initiating with tissue factor (TF).
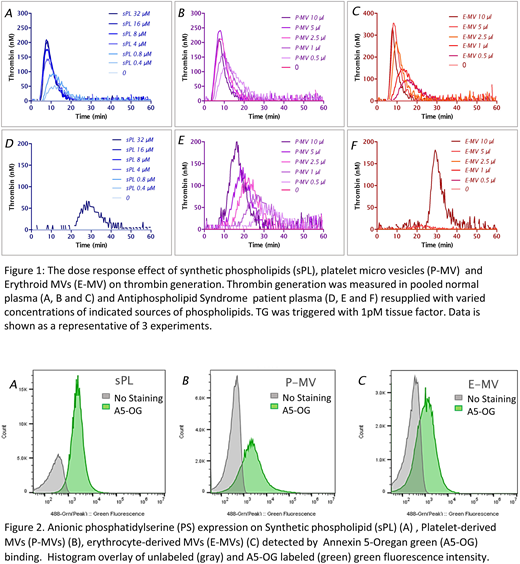
Results: P-MVs and sPL were equivalent as a source of PL in supporting TG in normal platelet poor plasmas. TG in pooled normal plasma (PNP) in the presence of 1 pM TF showed that P-MVs in dose-dependent fashion support thrombin generation to a similar degree as standard sPLs dilutions including a dose of 4 µM, validated earlier as a saturated concentration in CAT assay (Gerotziafas GT, et. al., 2005). Increasing concentration of either P-MVs or sPLs had little effect on lag time in PNP. In APS patient plasma, lag time was dose-dependently shortened with P-MVs, from 20 minutes to less than 10 minutes, and peak height was dose-dependently increased to a nearly normal level with the highest P-MVs tested. When sPLs was used, TG more severely affected. Strong thrombin generation occurred only with high concentration sPLs (32 µM) after more than 20 min lag time (Figure 1B). E-MVs showed a similar pattern in generating thrombin as sPL in normal plasma and in APS patient with prolonged lag time. The reversal of LAC phenomenon with correction of lag time in APS patients with P-MVs in TG CAT assay was confirmed by testing another 6 (out of 10) APS patients. Microvesicles size, analyzed on A60-Micro Plus, using large angle light scatter (LALS) demonstrated that all three types of MV were consistently less than 1micrometer in diameter. E-MVs and sPL had a similar size distribution, and the majority were of a diameter comparable to 110nm polystyrene calibration beads. P-MVs had wider distribution ranging up to 500nm. All three types of vesicles showed a PS expression by A5-OG labeling with 88.8%, 69.3% and 43.9% MV positivity for sPL, P-MVs and E-MVs, respectively and equivalent mean fluorescent intensity.
Summary: Platelet-derived microvesicles significantly promoted thrombin generation in APS patients. This is a revisiting of the "platelet neutralization effect" that was observed nearly 4 decades ago (Thiagarajan P, et. al., 1980). Our data demonstrate that P-MVs consistently increased TG in APS patient plasma compared to equivalent doses of sPL despite similar level of PS expression. In conclusion, cell-derived membranes from different source expressing PS might have different mechanisms to facilitate assembly of coagulation enzyme-substrate-cofactor complexes with resulting enzymatic activity than sPL and help explain LAC phenomenon.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal